Fluorine is a typical non-metal in the 10th cell of the periodic system table. As the one with the highest electronegativity of all the elements, fluorine is very active. Fluorine (F) is an element you encounter daily, most often as fluoride in water and toothpaste.
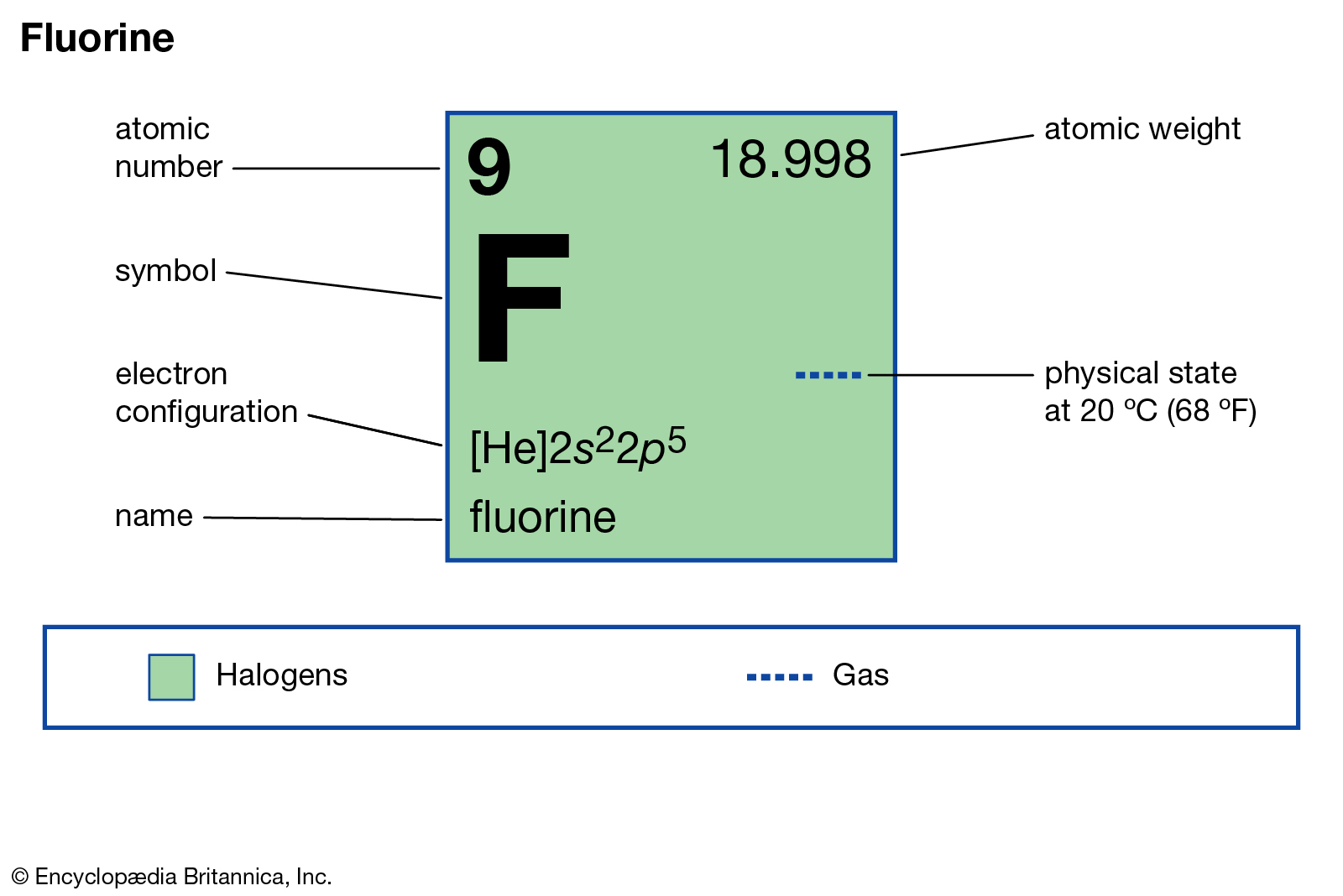 The position of the element fluorine in the periodic table
The position of the element fluorine in the periodic table
6 Interesting Facts about Fluorine
1. Fluorine is derived from the Latin word "Fluere" which signifies "Stream" or "Flux".
2. Fluorine was once known as a chemist killer. Fluorine is a highly reactive element and efforts to isolate it proved dangerous to chemists who attempted to isolate it. Chemists injured or killed by the search for pure fluorine were known as “Fluorine Martyrs”.
 Fluorine gas is so reactive it will ignite anything it touches.
Fluorine gas is so reactive it will ignite anything it touches.
3. It is difficult to store fluorine as it is corrosive to most metals.
4. Fluorine is rare in the universe, at only 400 parts per billion.
5. The mineral fluorite, or fluorspar, glows in the dark when exposed to light. This is where the term fluorescence comes from. It turns out, fluorine has nothing to do with this property. Small amounts of europium in fluorite causes this effect.
 Sodium fluoride is the additive in toothpaste and drinking water for preventing cavities in teeth.
Sodium fluoride is the additive in toothpaste and drinking water for preventing cavities in teeth.
6. Hydrofluoric acid, HF, dissolves glass. Its fluoride ions have a high affinity for calcium and can cause death by interfering with the body’s blood calcium metabolism when absorbed through the skin.


.jpg)