Search equation
Please enter the reactant or product to start the search
2Na3PO4 + 3CaCl2 → Ca3(PO4)2 + 6NaCl | , Phản ứng trao đổi
Na3PO4 | sodium phosphate | solid + CaCl2 | calcium chloride | solid = Ca3(PO4)2 | calcium phosphate | solid + NaCl | sodium chloride | solid | Temperature: temperature, Other Condition excess chlorine
Introduce
-
Detailed information about the equation
Reaction conditions when applied Na3PO4 + CaCl2
-
Detailed information on the reactants
-
Detailed information about the products of the reaction
Detailed information about the equation
Reaction conditions when applied Na3PO4 + CaCl2
- Catalyst: not available
- Temperature: normal
- Pressure: normal
- Other conditions: not available
Reaction process Na3PO4 + CaCl2
Process: updating...
Note: not available
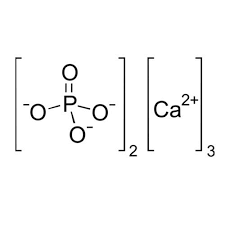
The result of the reaction Na3PO4 + CaCl2
The phenomenon: White precipitate of Ca3(PO4)2 appears in solution
Detailed information on the reactants
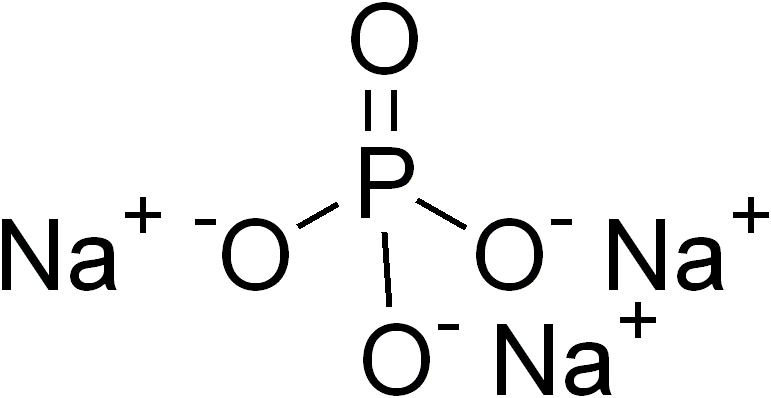
Information about Na3PO4 (sodium phosphate)
- Atomic weight: 163.9407
- Color: tinh thể hay có dạng hạt màu trắng
- Status: chất rắn
Information about CaCl2 (calcium chloride)
Detailed information about the products of the reaction
Information about Ca3(PO4)2 (calcium phosphate)
Information about NaCl (sodium chloride)
- Atomic weight: 58.4428
- Color: kết tinh màu trắng hay không màu
- Status: Chất rắn

Total rating:
Rating: / 5 star
The equations for preparation Na3PO4
Catalyst
normal
Temperature
normal
Pressure
normal
Other conditions
normal
Catalyst
normal
Temperature
normal
Pressure
normal
Other conditions
normal
Catalyst
normal
Temperature
normal
Pressure
normal
Other conditions
normal
The equations for preparation CaCl2
Catalyst
normal
Temperature
normal
Pressure
normal
Other conditions
normal
Catalyst
normal
Temperature
room temperature
Pressure
normal
Other conditions
normal
Catalyst
normal
Temperature
normal
Pressure
normal
Other conditions
normal
Interesting facts about chemistry you may not know
Interesting facts about hydrogen - the lightest element in the periodic table.
Hydrogen is the first element in the periodic system table. Hydrogen is known to be the lightest of all, the most abundant in the Universe, the essential element for life
View moreInteresting facts about helium
Helium is the first rare gas element in the periodic system table. In the Universe, it ranks second in abundance after elemental hydrogen.
View moreInteresting facts about lithium
Lithium is the alkali metal element, located in the third cell in the periodic table system. Lithium is the lightest of all solid metals and can cut a knife.
View moreInteresting Facts About Beryllium
Beryllium is the lightest alkaline earth metal. Beryllium is found in precious stones such as emeralds and aquamarine. Beryllium and its compounds are both carcinogenic.
View moreInteresting Facts About Carbon
Carbon is the non-metallic element in the sixth cell in the periodic system table. Carbon is one of the most important elements in all life, it is also known as the back.
View more

.jpg)