Gallium is the 31st cell element in the Periodic System Table predicted by Russian chemist and inventor Dimitri Mendeev, who created the periodic table of elements. He named the missing element eka - aluminum because he knew it would fall below aluminum in the Periodic Table in cell 31. Here are 10 interesting facts about gallium - the element named after the French rooster.
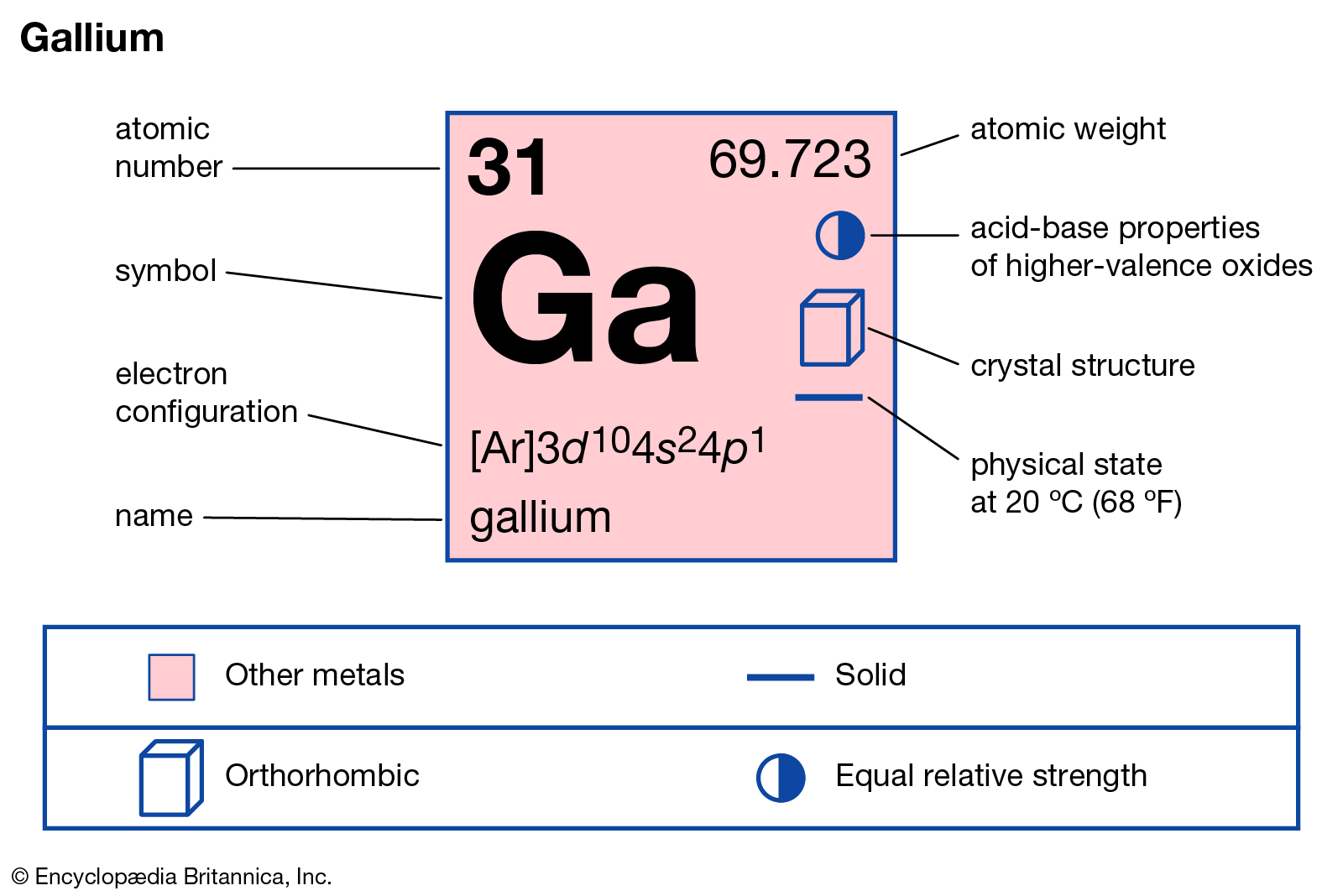
Location of gallium in the periodic system table.
10 Interesting Facts about Gallium
1. In 1875, Lecoq De Boisbaudran discovered and isolated pure gallium from two purple spectral lines in a sphalerite sample by electrolysis of hydroxides. However, the chemist Dmitri Mendeleev predicted the existence of the element in 1871, proposing the element's name "eka - aluminum" from its position in the periodic table.
.jpg)
Gallium is an iridescent silver metal.
2. Lecoq De Boisbaudran named this 31st element "gallia" in honor of his homeland, France. Gallia is the Latin name for Gaul. Some also say Lecoq De Boisbaudran's name is a play on words, naming his own element as well as France. "Le coq" is the French word meaning "rooster", while the Latin word for "rooster" is "gallus". The element is then named gallium after the -ium suffix for metallic elements.

Lecoq De Boisbaudran named this 31st element "gallia" in honor of his hometown of France, Gallia being the Latin name for Gaul. "Le coq" is the French word meaning "rooster", while the Latin word for "rooster" is "gallus".
3. In fact, Gallium has no role in human biology as well as animals and plants. It is a pure metal that is considered non-toxic.
4. In Medicine, gallium is used to monitor inflammation and mark tumors. This element has anti-cancer properties, especially against non-Hodgkin lymphoma and carcinoma. The gallium amine - phenol compound is very toxic to malaria parasites, including those that are resistant to chloroquine.
5. Gallium can be melted easily in the palm of the hand or hot beverage.
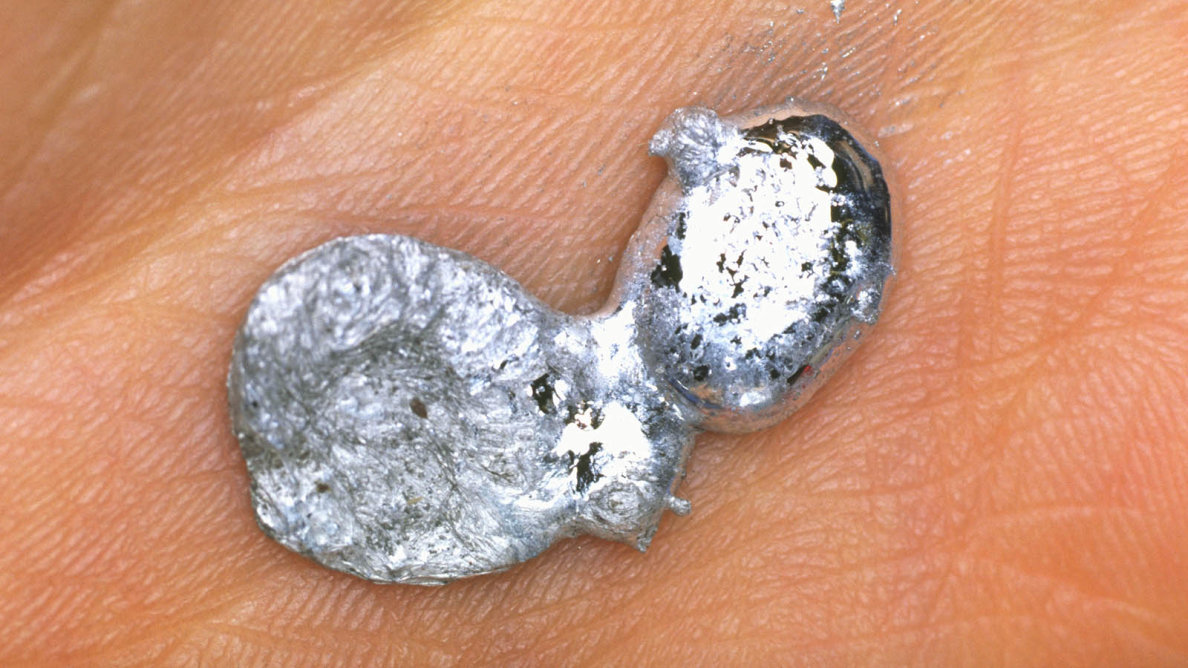
Gallium can be melted easily in the palm of the hand or hot beverage.
6. Gallium will expand when it freezes. Gallium should not be stored in metal or glass containers, as they can break when gallium thickens.
7. The Neutrino Observatory in Italy uses a large amount of gallium trichloride to study neutrinos produced in the sun. Neutrinos are particles created in the first second of the universe - even before atoms - and continuously generated through the nuclear reactions of the sun and other stars.

The Neutrino Observatory in Italy uses a large amount of gallium trichloride to study the neutrinos produced in the sun.
8. Liquid gallium forms a special mirror because the element can scan many surfaces. Gallium permeates glass, dolphins, and even human skin.
9. Gallium arsenide is used to produce ultra-bright blue LEDs.
10. More than 95% of gallium is used in the semiconductor industry. Pure gallium resists the attack of mineral acids. The oxide layer formed on the surface protects the interior.
More than 95% of gallium is used in the semiconductor industry.