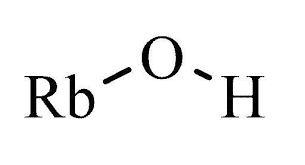Search equation
Please enter the reactant or product to start the search
RbOH.H2O → H2O + RbOH | Phương Trình Phản Ứng Hóa Học
RbOH.H2O | Rubidium hydroxide monohydrate | solid = H2O | water | solid + RbOH | Rubidium hydroxide; Rubidium hydoxide | solid | Temperature: temperature, Other Condition excess chlorine
Introduce
Detailed information about the equation
Reaction conditions when applied RbOH.H2O
- Catalyst: not available
- Temperature: 300
- Pressure: normal
- Other conditions: not available
Reaction process RbOH.H2O
Process: updating...
Note: not available
The result of the reaction RbOH.H2O
The phenomenon: updating...
Detailed information on the reactants
Information about RbOH.H2O (Rubidium hydroxide monohydrate)
Detailed information about the products of the reaction
Information about H2O (water)
Information about RbOH (Rubidium hydroxide; Rubidium hydoxide)
Total rating:
Rating: / 5 star
The equations for preparation RbOH.H2O
Catalyst
normal
Temperature
47 - 54
Pressure
vacuum
Other conditions
normal
Interesting facts about chemistry you may not know
Interesting facts about hydrogen - the lightest element in the periodic table.
Hydrogen is the first element in the periodic system table. Hydrogen is known to be the lightest of all, the most abundant in the Universe, the essential element for life
View moreInteresting facts about helium
Helium is the first rare gas element in the periodic system table. In the Universe, it ranks second in abundance after elemental hydrogen.
View moreInteresting facts about lithium
Lithium is the alkali metal element, located in the third cell in the periodic table system. Lithium is the lightest of all solid metals and can cut a knife.
View moreInteresting Facts About Beryllium
Beryllium is the lightest alkaline earth metal. Beryllium is found in precious stones such as emeralds and aquamarine. Beryllium and its compounds are both carcinogenic.
View moreInteresting Facts About Carbon
Carbon is the non-metallic element in the sixth cell in the periodic system table. Carbon is one of the most important elements in all life, it is also known as the back.
View more